The keyword question is straightforward: what does a rotary evaporator do? In one sentence, a rotary evaporator (often called a rotovap) removes and recovers solvents from a liquid mixture faster and at lower temperatures than many traditional methods—helping protect heat‑sensitive samples.
The simple answer: what does a rotary evaporator do?
A rotary evaporator removes a volatile solvent (like ethanol, acetone, or other lab solvents) from a solution and collects that solvent in a separate receiving flask. The goal is usually to concentrate a sample, exchange solvents, or recover expensive solvents for reuse.
In practice: the sample sits in a rotating flask inside a warm water (or oil) bath, while a vacuum pump reduces pressure so the solvent boils at a lower temperature. Vapor travels through a condenser, turns back into liquid, and drips into the receiving flask.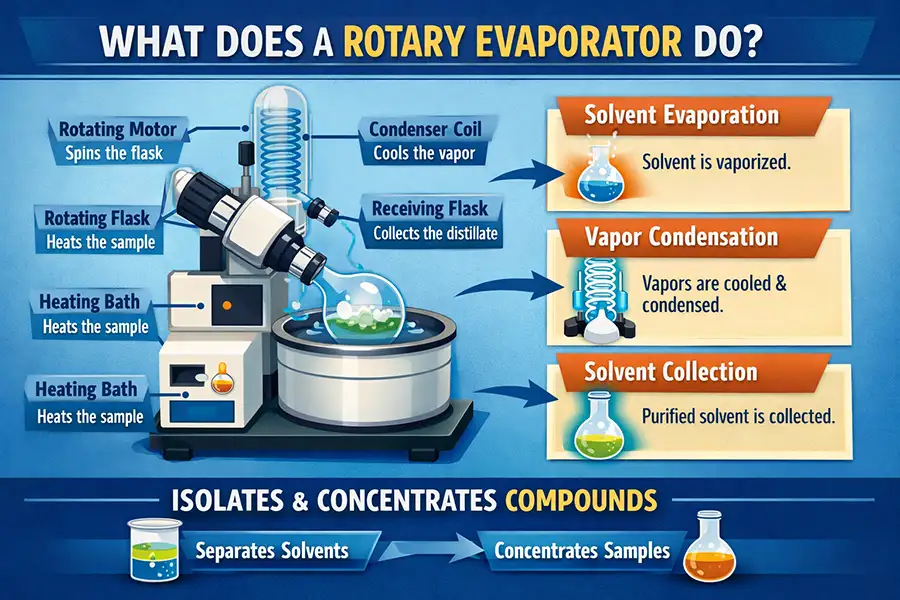 Image placeholder: a simple overview diagram showing flask rotation, bath heating, vacuum, condenser, and solvent recovery.Interactive question: Is a rotary evaporator just “distillation”?It’s related, but the purpose is usually different. Classic distillation often separates components by boiling points and can be run at atmospheric pressure. A rotovap is optimized for rapid solvent removal under vacuum, typically to concentrate a solution without overheating it.
Image placeholder: a simple overview diagram showing flask rotation, bath heating, vacuum, condenser, and solvent recovery.Interactive question: Is a rotary evaporator just “distillation”?It’s related, but the purpose is usually different. Classic distillation often separates components by boiling points and can be run at atmospheric pressure. A rotovap is optimized for rapid solvent removal under vacuum, typically to concentrate a solution without overheating it.
How a rotovap works (in 3 easy steps)
1) Lower the boiling point with vacuum
Vacuum reduces the pressure above the liquid, which reduces the temperature needed for the solvent to boil. That’s why rotary evaporation is often described as “gentle.”
2) Spread the liquid into a thin film by rotation
The rotating flask constantly spreads the sample into a moving film on the glass wall. A thinner film means more surface area, so solvent molecules escape faster.
3) Condense vapor and collect it
Solvent vapor moves into the condenser (often a vertical double-coil or double condenser design) and cools back into liquid. The condensed solvent is collected in a receiving flask—cleaner, easier to measure, and often reusable.
 Image placeholder: close-up of condenser and receiving flask for solvent recovery.
Image placeholder: close-up of condenser and receiving flask for solvent recovery.
Want an even deeper “how it works” explanation with setup tips? A related internal guide is here: How to Use a Rotary Evaporator.
Why rotation matters more than most people expect
If boiling is what removes solvent, why rotate at all? Because rotation helps prevent “bumping” (sudden violent boiling), improves heat transfer, and increases evaporation speed.
Interactive question: What happens if rotation is too fast?Too much speed can cause splashing, pushing sample toward the vapor path and contaminating the condenser. The practical solution is to start slow, pull vacuum gradually, then increase rotation only until the liquid forms a stable film.
Many benchtop models operate around 0–120 rpm (a common stepless regulation range), which gives flexibility across water-like solvents and thicker mixtures.
What problems does a rotary evaporator solve?
People searching “what does a rotary evaporator do” usually want practical outcomes. Here are the most common ones:
-
Concentration: remove 80–95% of solvent to make the remaining solution more concentrated.
-
Solvent recovery: capture ethanol/acetone/etc. instead of venting it—lower cost and less waste.
-
Solvent exchange: remove one solvent and replace it with another for the next process step.
-
Gentle handling: reduce thermal damage for flavors, fragrances, natural extracts, and sensitive intermediates.
For context on how widely vacuum is used in laboratories (including evaporation processes), the American Chemical Society (ACS) notes that vacuum techniques are foundational across chemistry labs for removing volatiles and drying—highlighting why efficient, controlled evaporation equipment remains a lab staple (ACS, educational resources on laboratory vacuum and solvent removal).
Key specs that actually matter
Specs can look overwhelming, so focus on the ones that directly affect daily results: flask volume, evaporation rate, maximum vacuum, bath temperature range, and condenser efficiency.
|
Series / Model |
Evaporating Flask (L) |
Receiving Flask (L) |
Rotation (rpm) |
Bath Temp Range |
Evaporation Rate (H₂O) |
Max Vacuum |
|
RE-201D |
2 |
1 |
0–120 |
RT–399°C |
> 1 L/h |
<133 Pa (≈1.33 mbar, 1 Torr) |
|
RE-301 |
3 |
2 |
0–120 |
RT–399°C |
> 1.5 L/h |
<133 Pa (≈1.33 mbar, 1 Torr) |
|
RE-501 |
5 |
3 |
0–120 |
RT–399°C |
> 1.5 L/h |
<133 Pa (≈1.33 mbar, 1 Torr) |
|
RE-1002 |
10 |
5 |
0–120 |
RT–99(250)°C |
> 3 L/h |
(vacuum depends on pump setup) |
|
RE-2002 |
20 |
10 |
0–90 |
RT–99(250)°C |
> 5 L/h |
(vacuum depends on pump setup) |
|
RE-5002 |
50 |
20 |
0–90 |
RT–99(250)°C |
> 9 L/h |
(vacuum depends on pump setup) |
← Swipe Left and Right to View the Table→
Looking for product options by capacity? Browse: Rotary Evaporator.
 Image placeholder: a lab bench rotovap setup showing bath, motor head, condenser, and receiving flask.
Image placeholder: a lab bench rotovap setup showing bath, motor head, condenser, and receiving flask.
How to choose the right rotary evaporator
Selection becomes easy if it’s based on a few questions:
-
Batch size: How many liters are processed per run? Choose a flask size with comfortable headspace.
-
Speed needs: Is the priority fast turnaround? Evaporation rate and condenser design matter.
-
Solvent type: Low-boiling solvents (e.g., acetone) are easier; water needs better vacuum and condenser cooling.
-
Workflow: Is frequent sample changeover needed? Lifting mechanism and ergonomic design matter.
Interactive question: Does a stronger vacuum always mean faster evaporation?Not always. Vacuum helps, but evaporation is also limited by condenser cooling capacity and how well the sample forms a thin film. In real labs, the “best” setup balances vacuum level, bath temperature, rotation speed, and condenser temperature to avoid bumping and losses.
Safety & best practices
-
Ramp vacuum slowly to prevent bumping and sample loss.
-
Do not overfill the evaporating flask; leave headspace for stable film formation.
-
Use compatible seals (commonly PTFE + fluoro rubber) for chemical resistance and vacuum stability.
-
Keep cooling steady so vapor condenses efficiently and doesn’t reach the pump.
-
Know the solvent (flammability, toxicity) and follow ventilation and PPE rules.
A rotary evaporator is one of those tools that feels “advanced,” but the idea is simple: remove solvent gently and efficiently. Once the balance of vacuum + rotation + heat + condensation is understood, it becomes a reliable daily workhorse.

 Products
Products







 Image placeholder: a simple overview diagram showing flask rotation, bath heating, vacuum, condenser, and solvent recovery.Interactive question: Is a rotary evaporator just “distillation”?It’s related, but the purpose is usually different. Classic distillation often separates components by boiling points and can be run at atmospheric pressure. A rotovap is optimized for rapid solvent removal under vacuum, typically to concentrate a solution without overheating it.
Image placeholder: a simple overview diagram showing flask rotation, bath heating, vacuum, condenser, and solvent recovery.Interactive question: Is a rotary evaporator just “distillation”?It’s related, but the purpose is usually different. Classic distillation often separates components by boiling points and can be run at atmospheric pressure. A rotovap is optimized for rapid solvent removal under vacuum, typically to concentrate a solution without overheating it. Image placeholder: close-up of condenser and receiving flask for solvent recovery.
Image placeholder: close-up of condenser and receiving flask for solvent recovery.
 Image placeholder: a lab bench rotovap setup showing bath, motor head, condenser, and receiving flask.
Image placeholder: a lab bench rotovap setup showing bath, motor head, condenser, and receiving flask.










