Struggling with unstable drug formulations or poor powder properties? Need an efficient way to produce consistent pharmaceutical powders? We understand these challenges well in drug development and manufacturing.
Pharmaceutical spray drying is a continuous process that transforms a liquid feed into a dry particulate form. It is vital for producing drugs with improved solubility, stability, and handling characteristics.
As a manufacturer with over 16 years of experience, I've seen firsthand how spray drying technology has revolutionized pharmaceutical production. This method is not just about drying; it's about engineering particles to meet specific therapeutic needs. We've helped countless clients optimize their drug formulations using this technology, moving from challenging liquid states to stable, effective powders. It’s a cornerstone for creating many modern medicines, and its precision is key.
What Exactly is Spray Drying in Pharmaceuticals?
Confused about how liquid medicine becomes a fine powder? This process might seem complex, but it's a smart solution for many pharmaceutical challenges. Let's break it down.
Spray drying in pharmaceuticals involves atomizing a liquid drug solution or suspension into fine droplets within a hot drying gas. This rapidly evaporates the solvent, leaving behind dry solid particles.

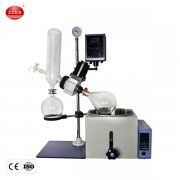
Going deeper, the spray drying process can be broken down into a few key stages. I always tell my team that understanding each stage is crucial for troubleshooting and optimizing the output for our clients. First, you have the feed preparation. This is where the active pharmaceutical ingredient (API) is dissolved or suspended in a suitable solvent. The choice of solvent and the concentration of the API are critical parameters here. It reminds me of a time when a client was struggling with particle agglomeration; we traced it back to an issue in their feed preparation. Adjusting the feed viscosity solved it completely. Next is atomization. The liquid feed is sprayed through a nozzle or a rotary atomizer, creating a vast surface area of fine droplets. The type of atomizer affects droplet size, which in turn influences the final particle characteristics. Then comes the drying phase. These droplets enter a drying chamber where they meet a hot stream of gas, usually air or nitrogen. The heat causes the solvent to evaporate almost instantly. The temperature and flow rate of this gas must be precisely controlled. Finally, there's particle collection. The dried particles are separated from the drying gas, typically using cyclones or bag filters. We often use spray drying techniques that ensure minimal product loss and high yield, which is so important in the pharmaceutical industry where materials are often very valuable.
Key Stages in Pharmaceutical Spray Drying:
|
Stage |
Description |
Critical Parameters |
|
Feed Preparation |
Dissolving or suspending the API in a solvent. |
API concentration, solvent type, viscosity, temperature. |
|
Atomization |
Converting the liquid feed into fine droplets. |
Nozzle type, feed rate, gas pressure/flow. |
|
Drying |
Evaporation of solvent from droplets using hot gas. |
Inlet/outlet gas temperature, gas flow rate, residence time. |
|
Particle Collection |
Separating dried particles from the drying gas. |
Cyclone efficiency, filter type, prevention of sticking. |
← Swipe Left and Right to View the Table→
Understanding these stages and parameters allows us, as manufacturers, to tailor the spray drying process for specific pharmaceutical applications, leading to optimized drug products. It’s this precision that makes our equipment so valuable to researchers and producers.
What Are the Key Advantages of Using Spray Dryers in Pharma?
Wondering if spray drying is the right choice for your pharmaceutical product? It offers unique benefits that can significantly improve drug manufacturing and performance. Let's explore why it's chosen.
Spray dryers offer rapid, continuous drying, control over particle size and morphology, enhanced drug stability, and improved solubility for poorly soluble APIs, making them highly advantageous in pharma.

When I talk to pharmaceutical companies, the advantages of spray drying often become the main topic. One major plus is speed. It’s a very fast process, turning a liquid into a dry powder in seconds. This rapid drying is great for heat-sensitive materials because the product itself doesn't get too hot for too long. I remember a project involving a delicate biologic; traditional drying methods like those used in a blast drying oven would have degraded it, but spray drying preserved its activity perfectly. Another big benefit is particle engineering. We can control the size, shape, and density of the particles. This is super important for how a drug behaves in the body, like how quickly it dissolves or how well it can be inhaled. We can create amorphous solid dispersions, which significantly boost the solubility of drugs that don't dissolve well in water. This is a game-changer for many new drug candidates. Also, the process is continuous, which means it's good for large-scale manufacturing once the process is set up. While some might ask is spray drying expensive, the efficiency and unique particle properties often justify the investment, especially for high-value pharmaceuticals. The ability to produce a dry, stable powder directly from a solution in a single step also simplifies the overall manufacturing workflow. It removes the need for other steps like milling or granulation in many cases.
Comparison of Drying Techniques:
|
Feature |
Spray Drying |
Freeze Drying (Lyophilization) |
Oven Drying |
|
Processing Time |
Fast (seconds to minutes) |
Slow (hours to days) |
Moderate to Slow (hours) |
|
Particle Control |
High control (size, morphology) |
Porous structure, less size control |
Limited control, often needs milling |
|
Heat Sensitivity |
Good for many heat-sensitive drugs (short exposure) |
Excellent (low temperature) |
Can degrade sensitive materials |
|
Solubility Enhancement |
Excellent (e.g., amorphous forms) |
Good (porous structure) |
Minimal |
|
Scalability |
Highly scalable, continuous |
Batch process, can be complex to scale |
Batch process, simple to scale |
← Swipe Left and Right to View the Table→
This ability to customize particle characteristics and handle sensitive compounds makes spray drying an invaluable tool in our industry. It’s not just about drying; it’s about creating better medicines.
How Do We Ensure Quality and Compliance in Pharmaceutical Spray Drying?
Worried about meeting strict pharmaceutical standards? Quality and compliance are non-negotiable when making medicines. We take this very seriously in our equipment design and process support.
Ensuring quality in pharmaceutical spray drying involves robust equipment design (e.g., GMP compliant), precise process control (PAT), rigorous validation, and adherence to regulatory guidelines like FDA and EMA.

From my 16 years in this business, I can tell you that quality is everything in pharmaceuticals. For spray drying, this starts with the equipment itself. Our spray dryers are built with materials like stainless steel that are easy to clean and don't react with the products. We design them to meet Good Manufacturing Practices (GMP) standards. This means things like smooth surfaces, no dead legs where material can get trapped, and systems for clean-in-place (CIP) or sterilize-in-place (SIP). Then there's process control. Modern spray dryers, like the ones we build, use Process Analytical Technology (PAT). This means we have sensors and systems that monitor critical process parameters in real-time – like inlet and outlet temperatures, feed rate, and airflow. This allows for immediate adjustments if anything starts to drift, ensuring consistent product quality. I often share a story about how a client using our PAT-enabled system caught a minor temperature fluctuation early, preventing a whole batch from going out of spec. Validation is another huge part. This involves proving that the spray drying process consistently produces a product meeting predetermined specifications. This includes Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). We guide our clients through this validation process. Finally, we always keep up with regulatory guidelines from bodies like the FDA and EMA. This ensures our equipment and the processes run on them are compliant. It's a continuous effort, but essential for patient safety and drug efficacy. Even for applications like producing specialized powders, similar to how a milk powder spray dryer operates under strict hygiene, pharmaceutical standards are even more stringent.
Key Aspects of Quality Assurance:
-
GMP-Compliant Design: Equipment materials, surface finish, ease of cleaning, and prevention of cross-contamination.
-
Process Validation (IQ/OQ/PQ): Documented evidence that the process consistently produces the desired product.
-
Process Analytical Technology (PAT): Real-time monitoring and control of critical process parameters.
-
Standard Operating Procedures (SOPs): Detailed instructions for equipment operation, maintenance, and cleaning.
-
Change Control Management: Formal process for managing any changes to the process or equipment.
-
Staff Training: Ensuring operators are fully trained on GMP principles and equipment operation.
By focusing on these areas, we help our pharmaceutical clients achieve the highest quality standards for their spray-dried products. It’s a responsibility we embrace fully.
What Are Common Applications of Spray Drying in Drug Manufacturing?
Curious about where spray drying truly shines in the pharmaceutical world? This versatile technology is used in a surprisingly wide range of drug development and production areas.
Common applications include producing amorphous solid dispersions for poorly soluble drugs, microencapsulation for controlled release, drying biologics, creating inhalable powders, and manufacturing taste-masked formulations.
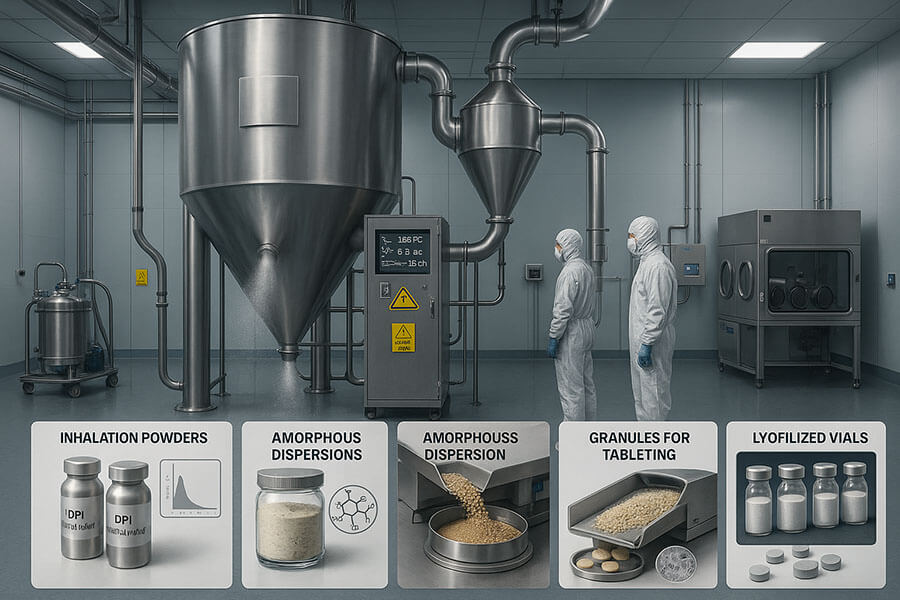
I've seen our spray dryers used for so many exciting applications in the pharmaceutical field. One of the most significant is creating amorphous solid dispersions (ASDs). Many new drug molecules are poorly soluble in water, which means they don't get absorbed well by the body. Spray drying can convert these crystalline drugs into an amorphous form, often mixed with a polymer, which dramatically improves their solubility and bioavailability. This has saved many promising drug candidates from being abandoned. Another key area is microencapsulation. We can use spray drying to coat drug particles with a polymer. This can be for taste masking – very important for pediatric medicines – or for creating controlled-release formulations that deliver the drug slowly over time. I recall a client developing a once-a-day version of an existing drug; spray drying was the key to achieving the desired release profile. Spray drying is also used for biologics, like proteins and peptides. These are often very delicate, but the rapid drying and controlled temperatures in a spray dryer can help maintain their stability and activity. For pulmonary drug delivery, spray drying can produce particles of the precise size and aerodynamic properties needed to be inhaled deep into the lungs. This is crucial for treating respiratory diseases. It's not just limited to these; we also see it used for drying extracts, producing tableting excipients, and even in some vaccine formulations. The versatility is truly remarkable, and as a manufacturer, it's rewarding to provide equipment that plays a role in so many different therapeutic innovations.
Examples of Pharmaceutical Applications:
|
Application Area |
Purpose |
Example Product Type |
|
Solubility Enhancement |
Improve dissolution of poorly water-soluble APIs. |
Amorphous Solid Dispersions (ASDs) for oral tablets/capsules. |
|
Controlled Release |
Modify drug release rate and duration. |
Microencapsulated particles for sustained-release oral drugs. |
|
Taste Masking |
Improve palatability of bitter APIs. |
Coated powders for pediatric suspensions or chewable tablets. |
|
Inhaled Therapies |
Produce fine powders for lung delivery. |
Dry powder inhalers (DPIs) for asthma or COPD. |
|
Biologics Stabilization |
Dry sensitive proteins, peptides, vaccines while preserving activity. |
Stable powder forms of biopharmaceuticals. |
← Swipe Left and Right to View the Table→
The adaptability of spray drying technology means it will continue to be a vital tool for pharmaceutical innovation, helping to bring more effective and patient-friendly medicines to the market.
Conclusion
Pharmaceutical spray drying is a powerful technique for creating high-quality drug powders. As experienced manufacturers, we see its vital role in advancing modern medicine daily.

 Products
Products