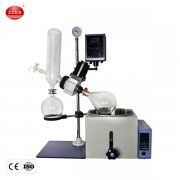
Distillation is a fundamental process in the chemical and pharmaceutical industries. It allows for the separation of components based on their boiling points, enabling the production of high-purity substances. One of the essential tools in this process is the Rotary Evaporator, commonly known as a Rotovap. In this article, we will explore the steps involved in making distillate with a Rotovap, highlighting its significance in various applications and rotovap machine price.
Understanding the Rotovap
Before delving into the distillation process, let's briefly understand what a Rotovap is and how it works. A Rotovap is a laboratory instrument used for efficient and gentle removal of solvents from samples by evaporation. It consists of a rotating flask, a heating bath, a condenser, and a vacuum system. This setup allows for precise control over temperature and pressure, making it an ideal choice for distillation processes.

Preparation of Sample
Before using the Rotovap, it's crucial to prepare the sample properly. This involves weighing the substance accurately and placing it in the rotating flask. The sample should be chosen based on the desired distillate, whether it's a chemical compound, essential oil, or any other substance.
Next, the solvent or mixture containing the desired compound is added to the flask. It's essential to ensure that the flask is not filled beyond its recommended capacity to allow for efficient evaporation.
Setting Up the Rotovap
Once the sample is prepared, the Rotovap needs to be set up correctly. This involves attaching the flask to the rotary evaporator, securing it tightly to prevent any leaks. The heating bath is then filled with an appropriate heat transfer fluid, and the condenser is connected to the cooling system.

Applying Heat and Vacuum
To initiate the distillation process, the heating bath is gradually heated. As the solvent in the sample flask begins to evaporate, it rises into the condenser, where it is cooled and condensed back into a liquid. The use of a vacuum system lowers the boiling point of the solvent, allowing for gentler evaporation and minimizing the risk of thermal degradation of sensitive compounds.
Collection of Distillate
The condensed distillate collects in a separate flask connected to the condenser. This flask contains the purified substance, which has been separated from the solvent. The collection process continues until the desired amount of distillate has been obtained.

Analyzing and Utilizing the Distillate
Once the distillation process is complete, it's essential to analyze the purity and composition of the distillate. Various analytical techniques can be used to ensure that the desired compound has been successfully isolated. Depending on the application, the distillate can be further processed, tested, or utilized in various industries, such as pharmaceuticals, chemistry, and essential oil production.

Conclusion
In conclusion, the Rotary Evaporator, or Rotovap, is a valuable tool for making distillate in a controlled and efficient manner. It plays a vital role in the separation and purification of compounds, making it indispensable in various scientific and industrial processes. Understanding the steps involved in making distillate with a Rotovap is essential for researchers and professionals in fields where high-purity substances are required.

 Products
Products